Vapor pressure is a critical concept in the world of science that plays a vital role in various industries. In this blog post, we’ll dive into the science behind vapor pressure, its significance, and applications across different sectors. By the end of the article, you’ll have a solid understanding of this fascinating phenomenon.
What is Vapor Pressure
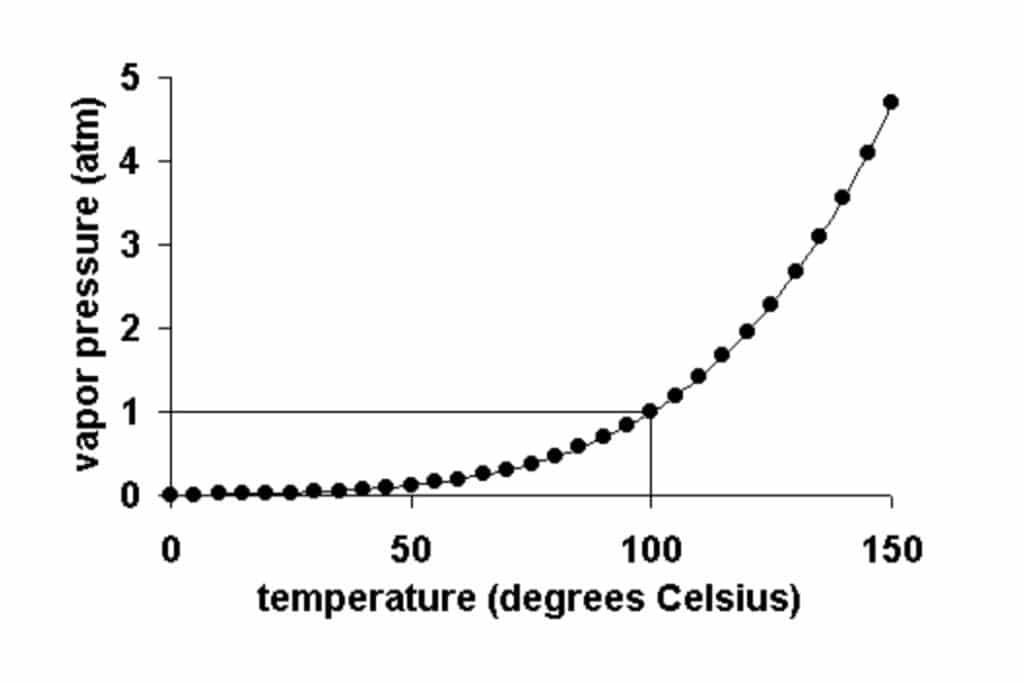
Vapor pressure is the pressure exerted by the gas phase of a substance when it’s in equilibrium with its liquid phase. In simpler terms, it’s the force generated by the evaporating molecules of a liquid at a given temperature. As temperature increases, the vapor pressure gaseous phase of a substance also rises, since more molecules gain enough energy to escape the liquid and enter the gas phase.
Why is Vapor Pressure Important
Understanding vapor pressure is crucial for several reasons:
Boiling Point Prediction
Vapor pressure helps us predict the boiling point of a substance. When the vapor pressure of a liquid equals atmospheric pressure, the liquid reaches its boiling point and starts to boil.
Phase Diagrams
Vapor pressure plays a significant role in creating phase diagrams, which are graphical representations of the different phases of a substance under varying conditions of temperature and pressure.
Chemical Stability
Vapor pressure is a critical parameter in determining the chemical stability of a substance. High vapor pressure indicates that a substance is volatile and prone to evaporation, whereas low vapor pressure implies that it’s less volatile and more stable.
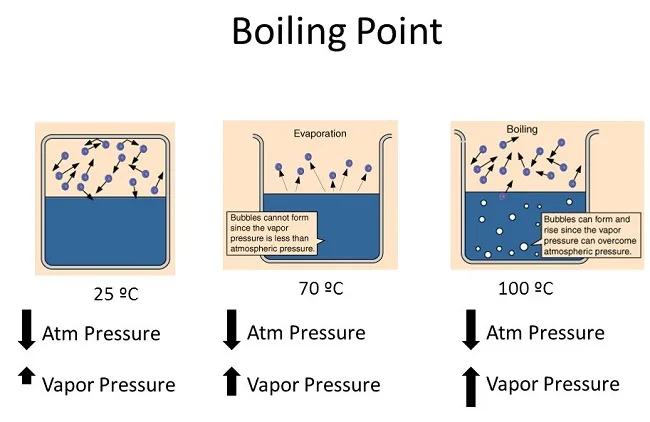
Normal boiling point
The normal boiling point of a substance is the temperature at which the substance changes from its liquid state to its gas state under a specific atmospheric pressure. This is usually measured under standard atmospheric pressure, which is defined as 1 atmosphere or 101.3 kilopascals. At this pressure, the boiling point of water is 100 degrees Celsius or 212 degrees Fahrenheit. Each substance has a different normal boiling point due to the unique intermolecular forces present in that substance.
Atmospheric pressure
Atmospheric pressure is the force per unit area exerted against a surface by the weight of the air above that surface in the Earth’s atmosphere. It’s also known as barometric pressure because barometers are used to measure it.
The standard or average atmospheric pressure at sea level on Earth is defined as 1 atmosphere (atm), which is equal to 101.3 kilopascals (kPa), 760 millimeters of mercury (mmHg) or torr, or 14.7 pounds per square inch (psi).
Atmospheric pressure changes with altitude. The higher you go above sea level, the less atmospheric pressure there is, because there’s less air above you exerting pressure. This is why it’s harder to breathe at higher altitudes; there’s less oxygen available in the air due to the decreased pressure.
It’s also worth noting that atmospheric pressure can vary with the weather. High-pressure systems generally bring clear skies and calm air, while low-pressure systems often bring clouds, wind, and precipitation.

Equilibrium vapor pressure
Equilibrium vapor pressure is a characteristic of a particular substance that describes the pressure exerted by its vapor when the substance is in a closed system at equilibrium, meaning the rate of evaporation equals the rate of condensation. This vapor pressure is equal to a state where the amount of substance in the liquid phase is constant over time.
The equilibrium vapor pressure of a substance is dependent on the temperature. As the temperature increases, more molecules have the necessary energy to escape the surface of the liquid and move into the gas phase, which increases the vapor pressure of the liquid.
Different substances have different equilibrium vapor pressures due to differences in intermolecular forces. Substances with strong intermolecular forces have lower equilibrium vapor pressures because more energy is needed to convert them from liquid to gas, while substances with weaker intermolecular forces have higher equilibrium vapor pressures.
For example, water has a relatively low equilibrium vapor pressure because its molecules are held together by strong hydrogen bonds. In contrast, ethanol, which has weaker intermolecular forces, has a higher equilibrium vapor pressure. The concept of equilibrium vapor pressure is crucial in understanding concepts like boiling point and volatility.
Applications of Vapor Pressure in Industries
Food and Beverage Industry
In the food and beverage industry, vapor pressure is essential for the preservation and quality control of products. For instance, controlling vapor pressure during the packaging process helps prevent spoilage and maintain the freshness of food items.
Pharmaceutical Industry
Vapor pressure plays a vital role in drug formulation and storage. For example, medications that are sensitive to humidity need to be stored in a controlled environment with low vapor pressure to prevent degradation of the active ingredients.
Petroleum Industry
In the petroleum industry, vapor pressure is used to determine the volatility of fuels and other petroleum products. This information helps engineers design safer storage and transportation systems for these volatile substances.
Frequently Asked Questions about Vapor Pressure
Q: How is vapor pressure measured?
A: Vapor pressure can be measured using various techniques, such as the static method, dynamic method, and effusion method. Each method has its advantages and limitations, depending on the substance being measured and the desired accuracy.
Q: How does temperature affect vapor pressure?
A: As mentioned earlier, temperature has a direct impact on vapor pressure. As temperature increases, more molecules gain enough energy to escape the liquid phase, resulting in a higher vapor pressure. This relationship with partial pressure is described by the Clausius-Clapeyron equation, which helps us calculate the vapor pressure of a substance at different temperatures.
Q: Are there any new developments or trends related to vapor pressure?
A: With advancements in technology, researchers are continually refining methods for measuring and predicting vapor pressure. For instance, computational methods, such as molecular modeling, are being employed to study the vapor pressures of complex substances and mixtures more accurately.
Q: What is vapor pressure in simple words?
A: Vapor pressure is the force exerted by the gas phase of a substance when it’s in equilibrium with its liquid phase. It’s the pressure generated by the evaporating molecules of the vapor phase of a liquid at a given temperature.
Q: What does it mean to have a high vapour pressure?
A high vapor pressure means that a substance is volatile and readily evaporates, as more molecules have enough energy to escape the liquid phase and enter the gas phase.
Q: What is vapor pressure and why is it important?
A: Vapor pressure is the pressure exerted by the gas phase of a substance in equilibrium with its liquid phase. It’s important because it helps predict boiling points, create phase diagrams, and determine chemical stability.
Q: What does vapor pressure determine?
A: Vapor pressure determines the volatility of a substance, its boiling point, and plays a significant role in phase diagrams and chemical stability.
Q: What has low vapor pressure?
A: Substances with strong intermolecular forces, such as hydrogen bonding, have low vapor pressures, as fewer molecules can escape the liquid phase and enter the gas phase.
Q: What happens when water vapor and pressure lowers?
A: When vapor pressure lowers, it indicates that a substance is less volatile and more stable, with fewer molecules escaping the liquid phase and entering the gas phase.
Q: What does vapor pressure tell you?
A: Vapor pressure tells you the volatility of a substance, its boiling point, and provides information on chemical stability and phase diagrams.
Q: Does low vapor pressure mean low boiling point?
A: No, low vapor pressure means a higher boiling point since the substance requires more energy to reach a point where its vapor pressure equals atmospheric pressure.
Q: How does vapor pressure affect equilibrium?
A: Vapor pressure affects equilibrium as it represents the pressure at which the rates of evaporation and condensation are equal, establishing a dynamic equilibrium or balance between the liquid and gas phases.
Q: What is the formula of vapour pressure in equilibrium?
A: The Clausius-Clapeyron equation describes the relationship between vapor pressure and temperature: ln(P2/P1) = ΔHvap/R(1/T1 – 1/T2), where P1 and P2 are vapor pressures at temperatures T1 and T2, ΔHvap is the enthalpy of vaporization, and R is the gas constant.
Q: What causes equilibrium vapor pressure?
A: Equilibrium vapor pressure is caused by a balance between evaporation and condensation rates, with molecules continuously escaping the liquid phase and returning to it.
Q: How is vapour pressure equal to equilibrium constant?
A: Vapor pressure is not directly equal to the equilibrium constant, but both represent vapor pressure reaches a state of equilibrium. Vapor pressure refers to the pressure at which evaporation and condensation rates are equal, while the equilibrium constant represents the ratio of product concentrations to reactant concentrations at equilibrium.
Q: What is the meaning of vapour pressure?
A: Vapor pressure is the pressure exerted by the gas phase of a substance when it’s in equilibrium with its liquid phase, representing the force generated by evaporating molecules of a liquid at a given temperature.
Q: What is vapor pressure and what causes it?
A: Vapor pressure is the pressure exerted by the gas phase of a substance when it’s in equilibrium with its liquid phase. It’s caused by the evaporation of molecules from the liquid phase and their subsequent entry into the gas phase.
Q: Does higher vapor pressure mean higher vapor pressure curve too?
A: Higher vapor pressure means higher volatility, but it doesn’t necessarily mean higher overall pressure, as the total pressure depends on other factors such as atmospheric pressure and the presence of other gases.
Q: What does a high or low vapor pressure mean?
A: A high vapor pressure means a substance is more volatile and prone to evaporation, while a low vapor pressure indicates that a substance is less volatile and more stable.
Q: How do you determine a higher temperature and vapor pressure?
A: Higher vapor pressure can be determined by comparing the vapor pressures of different substances at the same temperature, or by calculating the vapor pressure of a substance at various temperatures using the Clausius-Clapeyron equation.
Q: What has higher vapour pressure?
A: Substances with weaker intermolecular forces, such as London dispersion forces, have higher vapor pressures, as more molecules can escape the liquid phase and enter the gas phase.

Q: What are the factors affecting vapor pressure?
A: Factors affecting vapor pressure include temperature, intermolecular forces, and the presence of solutes in the liquid phase.
Q: What causes vapor pressure to increase?
A: Vapor pressure increases with increasing temperature, as more molecules gain enough energy to escape the liquid phase and enter the gas phase.
Q: What factor has the largest effect on vapor pressure?
A: Temperature has the largest effect on vapor pressure, as it directly influences the energy available for molecules to escape the liquid phase and enter the gas phase.
Q: What is meant by vapor pressure?
A: Vapor pressure is the pressure exerted by the gas phase of a substance when it’s in equilibrium with its liquid phase, representing the force generated by evaporating molecules of a liquid at a given temperature.
Q: Why does vapor pressure increase with temperature?
A: Vapor pressure increases with temperature because more molecules gain enough energy to escape the liquid phase and enter the gas phase, resulting in a higher pressure exerted by the gas.
Q: Does vapor pressure equal 1 atm?
A: Vapor pressure equals 1 atm when the boiling point of a substance is reached at standard atmospheric pressure (1 atm).
Q: What affects vapor pressure of a liquid?
A: Factors affecting vapor pressure of a liquid include temperature, intermolecular forces, and the presence of solutes in the liquid phase.
Q: What is vapour pressure in chemistry?
A: In chemistry, vapor pressure is the pressure exerted by the gas phase of a substance when it’s in equilibrium

Conclusion
Vapor pressure is a fascinating scientific concept with significant applications across various industries. Understanding and controlling vapor pressure is crucial for ensuring product quality, safety, and stability. By staying up to date with the latest developments and trends in this field, we can continue to harness the power of vapor pressure and improve our understanding of the world around us.