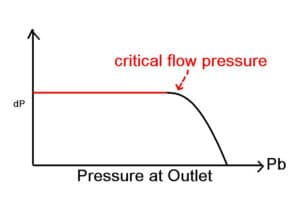
What is meant by Critical Pressure and Critical Temperature?
The critical pressure is the vapor pressure of a fluid at the critical temperature above which distinct liquid and gas phases do not exist. As the critical temperature is approached, the properties of the gas and liquid phases become the same, resulting in only one phase.
0 Comments